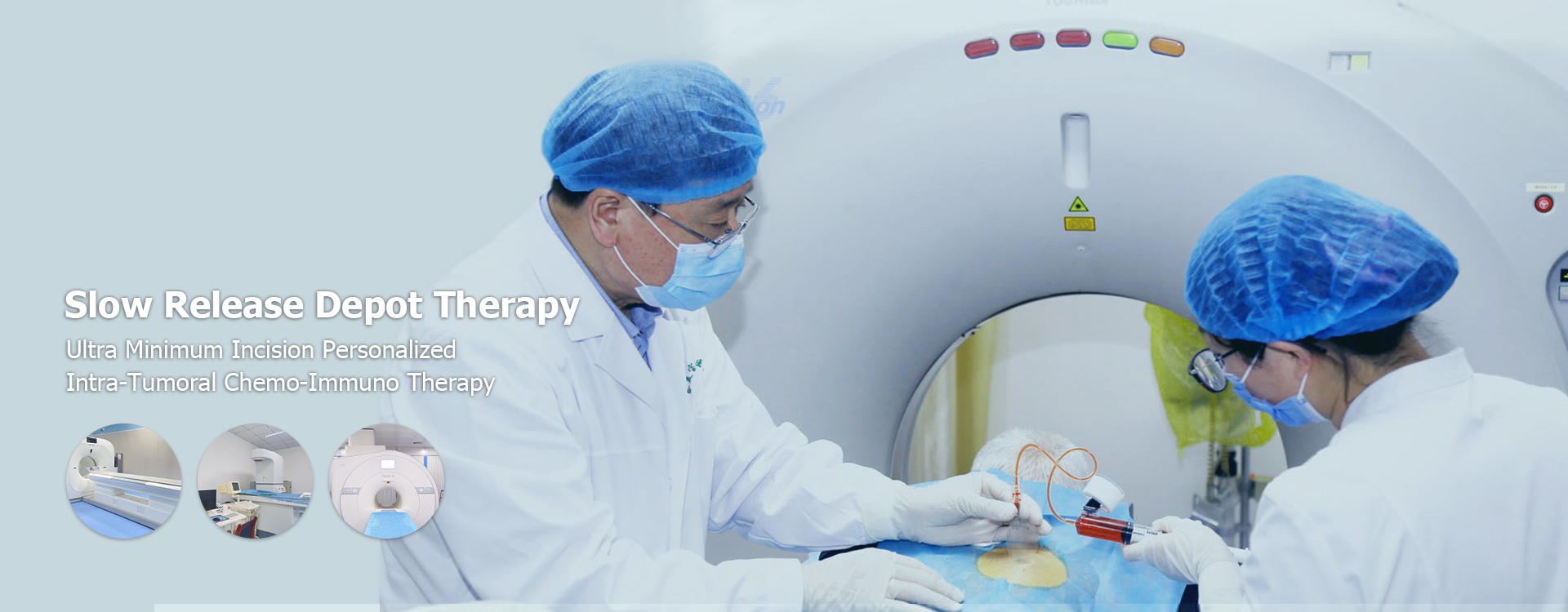
Ultra-Minimum Incision Personalized Intratumoral Chemoimmunotherapy: A Comprehensive Guide
2025-03-07
- Understanding Intratumoral Chemoimmunotherapy
- The ‘Ultra-Minimum Incision’ Approach
- Personalized Chemoimmunotherapy: Tailoring Treatment to the Individual
- The Procedure: Step-by-Step
- Potential Benefits of Ultra-Minimum Incision Personalized Intratumoral Chemoimmunotherapy
- Examples of Cancers Treated with Intratumoral Chemoimmunotherapy
- Clinical Trial Data and Research
- The Future of Intratumoral Chemoimmunotherapy
- Patient Selection and Considerations
- Potential Risks and Side Effects
- Cost and Accessibility
- Conclusion
Ultra-Minimum Incision Personalized Intratumoral Chemoimmunotherapy is an innovative cancer treatment approach combining minimally invasive surgery with personalized chemoimmunotherapy delivered directly into the tumor. This method aims to maximize treatment efficacy while minimizing side effects, potentially leading to improved patient outcomes. This article delves into the details of this technique, including its principles, procedures, benefits, and future directions.
Understanding Intratumoral Chemoimmunotherapy
Intratumoral chemoimmunotherapy involves directly injecting chemotherapeutic agents and/or immunotherapeutic agents into a tumor. This localized delivery allows for a higher concentration of the drug to reach the tumor site, reducing systemic exposure and toxicity compared to traditional intravenous chemotherapy. It is often used in conjunction with other treatments like surgery or radiation therapy.
The Evolution of Cancer Treatment
Traditional cancer treatments, such as systemic chemotherapy and radiation therapy, often affect the entire body, leading to significant side effects. Intratumoral therapy represents a shift towards more targeted and personalized approaches. By focusing treatment directly on the tumor, it aims to spare healthy tissues and improve the patient’s quality of life. Shandong Baofa Cancer Research Institute is committed to innovation in this field.
The ‘Ultra-Minimum Incision’ Approach
The ‘Ultra-Minimum Incision’ aspect refers to the surgical technique used to access the tumor for injection. This typically involves small incisions, often guided by imaging technologies such as ultrasound or CT scans, to ensure precise delivery of the therapeutic agents. The minimally invasive nature of the procedure contributes to faster recovery times and reduced post-operative pain.
Benefits of Minimally Invasive Surgery
- Smaller scars
- Less pain
- Shorter hospital stay
- Faster recovery
- Reduced risk of complications

Personalized Chemoimmunotherapy: Tailoring Treatment to the Individual
Personalization is a key element of this approach. Before treatment, the patient’s tumor is analyzed to identify its specific characteristics, such as genetic mutations and immune markers. This information is then used to select the most effective chemotherapeutic and immunotherapeutic agents for that particular tumor. This personalized approach maximizes the chances of treatment success and minimizes the risk of adverse reactions.
The Role of Biomarker Analysis
Biomarker analysis is crucial for personalized chemoimmunotherapy. By identifying specific biomarkers in the tumor, clinicians can predict which drugs are most likely to be effective. For example, PD-L1 expression can indicate the potential responsiveness to certain immunotherapies.

The Procedure: Step-by-Step
The Ultra-Minimum Incision Personalized Intratumoral Chemoimmunotherapy procedure typically involves the following steps:
- Pre-operative assessment: The patient undergoes a thorough evaluation, including imaging studies and biomarker analysis.
- Treatment planning: Based on the assessment, a personalized treatment plan is developed, specifying the chemotherapeutic and immunotherapeutic agents to be used.
- Procedure: The patient undergoes the minimally invasive procedure, during which the drugs are injected directly into the tumor under image guidance.
- Post-operative monitoring: The patient is closely monitored for any side effects or complications. Follow-up imaging studies are performed to assess the response to treatment.
Potential Benefits of Ultra-Minimum Incision Personalized Intratumoral Chemoimmunotherapy
This innovative approach offers several potential benefits compared to traditional cancer treatments:
- Improved efficacy: Higher concentration of drugs at the tumor site.
- Reduced toxicity: Lower systemic exposure to chemotherapy.
- Personalized treatment: Tailored to the individual patient’s tumor characteristics.
- Minimally invasive: Faster recovery and less pain.
- Potential for immune stimulation: Intratumoral delivery of immunotherapeutic agents can stimulate the immune system to attack the tumor.
Examples of Cancers Treated with Intratumoral Chemoimmunotherapy
While research is ongoing, Ultra-Minimum Incision Personalized Intratumoral Chemoimmunotherapy has shown promise in treating a variety of cancers, including:
- Melanoma
- Hepatocellular carcinoma (Liver Cancer)
- Breast Cancer
- Lung Cancer
- Other solid tumors
Clinical Trial Data and Research
Numerous clinical trials are underway to evaluate the safety and efficacy of Ultra-Minimum Incision Personalized Intratumoral Chemoimmunotherapy. Early results are promising, with some studies showing significant tumor regression and improved survival rates in patients treated with this approach. For instance, a study published in the Journal of Clinical Oncology demonstrated a significant improvement in overall survival for patients with advanced melanoma treated with intratumoral immunotherapy compared to those treated with systemic chemotherapy [1].
The Future of Intratumoral Chemoimmunotherapy
The field of intratumoral chemoimmunotherapy is rapidly evolving. Future directions include:
- Development of new and more effective chemotherapeutic and immunotherapeutic agents.
- Improved methods for personalizing treatment based on tumor characteristics.
- Combination of intratumoral therapy with other cancer treatments, such as radiation therapy and targeted therapy.
- Use of nanotechnology to deliver drugs more precisely to the tumor site.
Patient Selection and Considerations
Not all patients are suitable candidates for Ultra-Minimum Incision Personalized Intratumoral Chemoimmunotherapy. Factors to consider include:
- Tumor size and location
- Patient’s overall health
- Previous cancer treatments
- Presence of specific biomarkers
A thorough evaluation by a multidisciplinary team of oncologists, surgeons, and other specialists is essential to determine whether this treatment approach is appropriate for a particular patient. Patients seeking more information are welcome to contact us for an initial consultation.
Potential Risks and Side Effects
As with any medical procedure, Ultra-Minimum Incision Personalized Intratumoral Chemoimmunotherapy carries some risks and potential side effects. These may include:
- Pain and swelling at the injection site
- Infection
- Bleeding
- Allergic reactions to the drugs
- Systemic side effects of chemotherapy
It is important to discuss these risks with your doctor before undergoing treatment.
Cost and Accessibility
The cost of Ultra-Minimum Incision Personalized Intratumoral Chemoimmunotherapy can vary depending on the specific drugs used, the complexity of the procedure, and the location of the treatment center. It is important to discuss the costs with your healthcare provider and insurance company. Accessibility may also be a factor, as this treatment is not yet widely available at all cancer centers.
Conclusion
Ultra-Minimum Incision Personalized Intratumoral Chemoimmunotherapy represents a promising new approach to cancer treatment. By combining minimally invasive surgery with personalized chemoimmunotherapy, it offers the potential to improve efficacy, reduce toxicity, and enhance the patient’s quality of life. While further research is needed, this innovative approach holds great promise for the future of cancer care. At Shandong Baofa Cancer Research Institute, we remain dedicated to pioneering advancements in cancer treatment and providing patients with the best possible care. Contact us to learn more about our comprehensive cancer treatment options.
References
[1] Ribas, A., et al. (2018). Intratumoral Immunotherapy. Journal of Clinical Oncology, 36(9), 919-927.