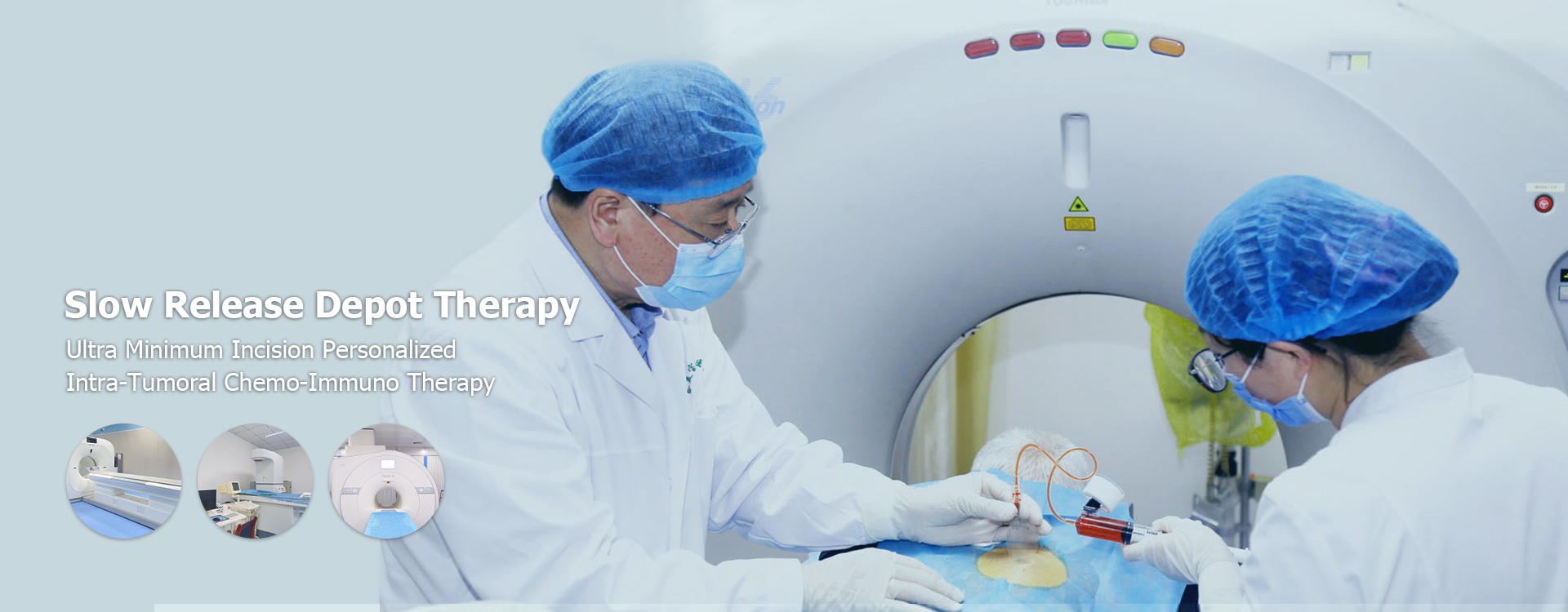
Controlled Release Drug Delivery cost
Controlled Release Drug Delivery Cost: A Comprehensive GuideUnderstanding the factors influencing the cost of controlled release drug delivery systems.
This article provides a comprehensive overview of the costs associated with controlled release drug delivery systems. We will explore the various factors that influence pricing, including research and development, manufacturing processes, regulatory approvals, and market demand. The information provided aims to offer a clear understanding of the financial considerations involved in this specialized area of pharmaceutical development. We will delve into different types of controlled release systems and analyze their respective cost structures.
Factors Affecting the Cost of Controlled Release Drug Delivery
Research and Development Costs
The initial phase of developing a controlled release drug delivery system involves substantial research and development (R&D) costs. This includes formulation development, pre-clinical testing, and the optimization of the release profile. The complexity of the delivery system significantly impacts the overall cost. More sophisticated systems, such as implantable pumps or injectable microspheres, often demand higher upfront investment in R&D compared to simpler systems like extended-release tablets.
Manufacturing Costs
Manufacturing costs vary greatly depending on the chosen delivery system and the scale of production. The complexity of the manufacturing process, the materials used, and the level of automation all play a role. For instance, the production of biodegradable polymers for implantable devices requires specialized equipment and expertise, resulting in higher manufacturing costs. Conversely, large-scale production of simple extended-release tablets can be more cost-effective. The need for sterile manufacturing processes for injectable formulations also increases the overall expense.
Regulatory Costs
Securing regulatory approvals is crucial for bringing any pharmaceutical product to market, and controlled release drug delivery systems are no exception. The regulatory pathway for these systems can be complex and lengthy, requiring significant investment in documentation, testing, and interactions with regulatory agencies like the FDA (in the US) or EMA (in Europe). These costs can vary depending on the specific regulatory requirements for the drug and the delivery system.
Market Demand and Competition
The overall market demand for a particular controlled release drug delivery system can influence its cost. High demand can lead to economies of scale, potentially reducing manufacturing costs. However, competition in the market also plays a significant role. A high level of competition can drive prices down, while a lack of competition can lead to higher prices.
Types of Controlled Release Drug Delivery Systems and Their Costs
Various types of controlled release drug delivery exist, each with its own cost profile. These include:
- Extended-release tablets and capsules: Generally, these are the most cost-effective option due to their relatively simple manufacturing process.
- Implantable pumps: These systems are significantly more expensive due to the complexity of their design, materials, and surgical implantation.
- Injectable microspheres and nanoparticles: These systems can offer advantages in terms of targeted drug delivery, but their manufacturing processes are often intricate and thus expensive.
- Transdermal patches: These systems offer a convenient method of drug administration but can have variable costs depending on the complexity of the adhesive and drug release mechanisms.
Cost Comparison Table
| Delivery System | Approximate Cost Range (USD) | Factors Influencing Cost |
|---|---|---|
| Extended-release tablets | $0.50 - $5 per dose | Manufacturing scale, excipients |
| Injectable microspheres | $50 - $500 per dose | Complexity of manufacturing, materials |
| Implantable pumps | $1000 - $10,000+ per device | Surgical procedure, device complexity, materials |
| Transdermal patches | $1 - $20 per patch | Patch size, drug concentration, adhesive type |
Note: The cost ranges provided are estimates and can vary significantly depending on several factors. Consult with pharmaceutical manufacturers for precise pricing information.
Conclusion
The cost of controlled release drug delivery is influenced by numerous interconnected factors. Understanding these factors is crucial for pharmaceutical companies, researchers, and healthcare providers involved in the development, selection, and utilization of these systems. Careful consideration of R&D, manufacturing, regulatory, and market dynamics is essential for optimizing both efficacy and affordability. For more detailed information on specific controlled release technologies and their applications, you may wish to contact Shandong Baofa Cancer Research Institute for further guidance.
Disclaimer: This information is for educational purposes only and should not be considered medical advice. Consult with a healthcare professional for any health concerns or before making any decisions related to your health or treatment.
Related products
Related products
Best selling products
Best selling products-
 Mark, a prostate cancer bone metastasis patient from the United States
Mark, a prostate cancer bone metastasis patient from the United States -
 Famous American female painter Muriel
Famous American female painter Muriel -
 Anthony, lymphocytic cancer patient from the United States 24
Anthony, lymphocytic cancer patient from the United States 24 -
 Nell Smith, a throat cancer patient from Switzerland
Nell Smith, a throat cancer patient from Switzerland -
 PAT, rectal cancer patient from the United States
PAT, rectal cancer patient from the United States -
 Andress, a 9-year-old boy from the United States
Andress, a 9-year-old boy from the United States
Related search
Related search- Cheap symptoms pancreatic cancer Hospitals
- treatment liver cancer survival near me
- China cribriform prostate cancer treatment near me
- treatment new treatments for lung cancer stage 4 cost
- China Gallbladder cancer
- Cheap signs of breast cancer
- pi rads 5 prostate cancer treatment
- treatment stage 3 lung cancer treatment Hospitals
- Sustained Release Drug Delivery Therapy
- Cheap rcc cost