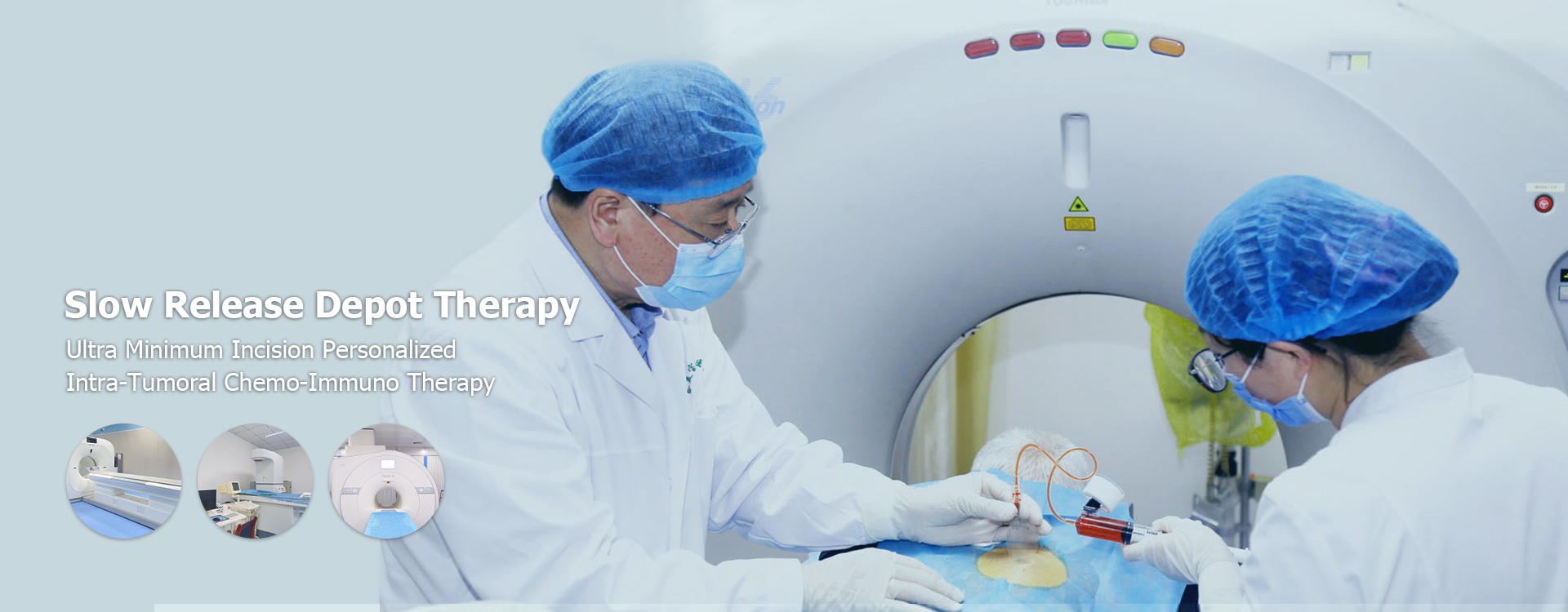
China Targeted Drug Delivery for Cancer Hospitals
China Targeted Drug Delivery for Cancer Hospitals
This article explores the advancements and challenges in China targeted drug delivery systems specifically designed for cancer hospitals. We'll examine current technologies, future trends, and the crucial role these systems play in improving cancer treatment efficacy and patient outcomes. Learn about various delivery methods, their applications, and the regulatory landscape within China's healthcare system.
Current Technologies in Targeted Drug Delivery for Cancer
Nanoparticle-Based Drug Delivery
Nanoparticles offer a promising approach to China targeted drug delivery for cancer hospitals. Their small size allows for enhanced penetration into tumor tissues, leading to increased drug accumulation at the target site and reduced systemic toxicity. Liposomes, polymeric nanoparticles, and inorganic nanoparticles are among the most researched and developed systems. Several clinical trials in China are evaluating the efficacy and safety of these nanoparticle-based drug delivery systems for various types of cancers. Further research is needed to optimize their biodistribution and minimize potential side effects.
Antibody-Drug Conjugates (ADCs)
ADCs represent another significant advancement in targeted cancer therapy. These conjugates link a cytotoxic drug to a monoclonal antibody that specifically binds to cancer cells. This targeted approach maximizes the drug's effect on cancerous cells while minimizing harm to healthy tissues. China's pharmaceutical industry is actively involved in developing and manufacturing ADCs, with several products undergoing clinical trials or already approved for specific cancer types. The continued development and refinement of ADCs are vital for improving treatment outcomes in China targeted drug delivery for cancer hospitals.
Other Targeted Delivery Methods
Beyond nanoparticles and ADCs, other methods are being explored, including gene therapy vectors, aptamer-drug conjugates, and targeted liposomes. Each approach offers unique advantages and challenges, and ongoing research continues to refine these techniques to enhance their efficacy and safety. The selection of the most appropriate delivery method depends on various factors, including the type of cancer, the drug's properties, and the patient's overall health.
Challenges and Future Directions
Regulatory Landscape in China
The regulatory pathway for new China targeted drug delivery systems in China can be complex. Strict guidelines and rigorous testing are necessary to ensure the safety and efficacy of these therapies. Collaboration between pharmaceutical companies, regulatory bodies, and research institutions is essential to streamline the approval process and accelerate the adoption of innovative targeted drug delivery technologies in Chinese cancer hospitals. The National Medical Products Administration (NMPA) plays a key role in overseeing this process.
Accessibility and Affordability
Ensuring accessibility and affordability of these advanced therapies is crucial for improving cancer care in China. Targeted drug delivery systems can be expensive, posing a challenge for many patients. Government initiatives and collaborations with pharmaceutical companies are vital to make these life-saving treatments more widely available to those who need them. The Shandong Baofa Cancer Research Institute is actively involved in exploring these solutions.
Personalized Medicine
The future of China targeted drug delivery for cancer hospitals lies in personalized medicine. Tailoring treatment plans to individual patients based on their unique genetic makeup and tumor characteristics will optimize therapeutic outcomes. Further research into biomarkers and predictive modeling will be critical to developing truly personalized targeted therapies.
Conclusion
China targeted drug delivery for cancer hospitals is a rapidly evolving field with immense potential to revolutionize cancer treatment. While challenges remain, ongoing research and development efforts are paving the way for safer, more effective, and more accessible targeted therapies. The collaboration between researchers, clinicians, regulatory bodies, and healthcare providers is crucial to ensuring that these advancements translate into improved patient outcomes across China.
Related products
Related products
Best selling products
Best selling products-
 PAT, rectal cancer patient from the United States
PAT, rectal cancer patient from the United States -
 Anthony, lymphocytic cancer patient from the United States 24
Anthony, lymphocytic cancer patient from the United States 24 -
 Andress, a 9-year-old boy from the United States
Andress, a 9-year-old boy from the United States -
 Mark, a prostate cancer bone metastasis patient from the United States
Mark, a prostate cancer bone metastasis patient from the United States -
 Famous American female painter Muriel
Famous American female painter Muriel -
 Nell Smith, a throat cancer patient from Switzerland
Nell Smith, a throat cancer patient from Switzerland
Related search
Related search- pancreatic cancer treatment near me
- Cheap best supplements for prostate cancer treatment Hospitals
- China 5 day radiation treatment for lung cancer
- Cheap best supplements for prostate cancer treatment
- China best prostate cancer treatment centers 2021 cost
- papillary renal cell carcinoma
- treatment Sustained Release Drug Delivery Therapy
- China average cost of lung cancer treatment
- treatment lung cancer treatment options near me
- treatment Cancer Hospital