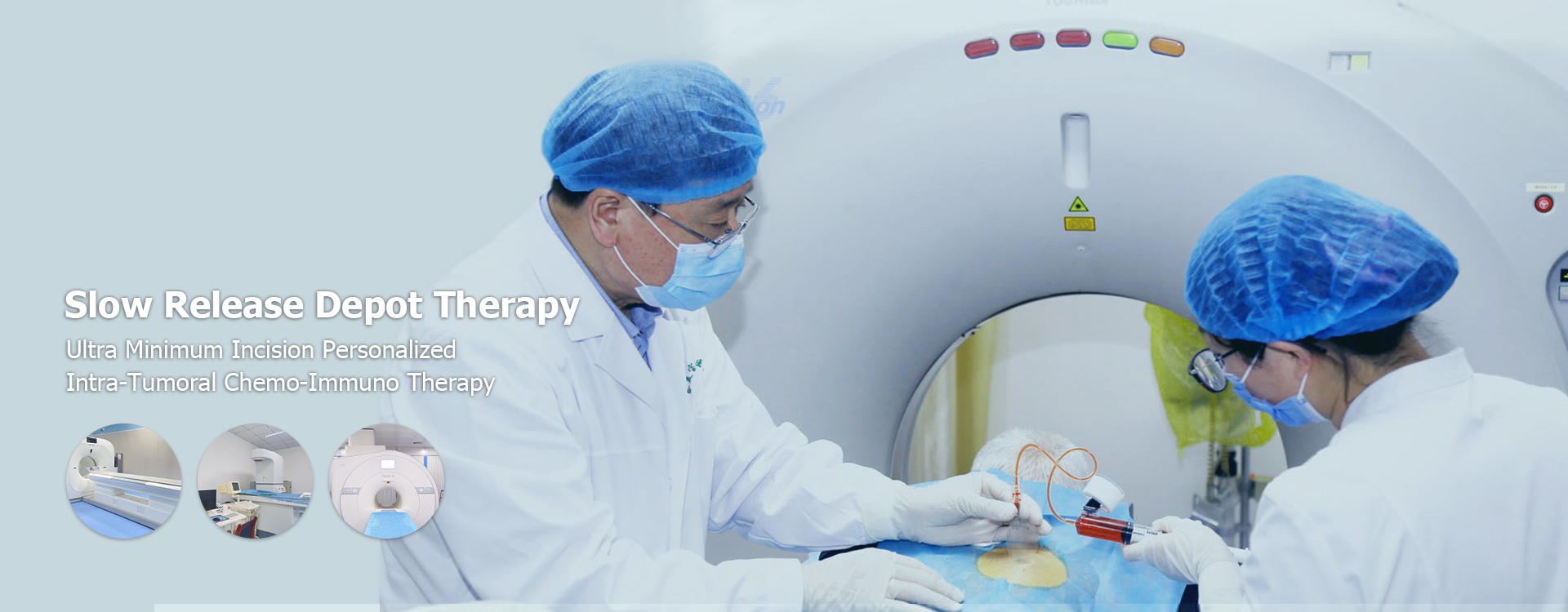
China Sustained Release Drug Delivery Therapy
China Sustained Release Drug Delivery Therapy: A Comprehensive Overview
This article provides a comprehensive overview of China Sustained Release Drug Delivery Therapy, exploring its advancements, applications, and future prospects. It examines the various technologies employed, regulatory landscape, and ongoing research in this crucial area of pharmaceutical development. Learn about the benefits, challenges, and potential impact on patient care.
Types of Sustained Release Drug Delivery Systems in China
Polymer-Based Systems
Polymer-based systems are widely used in China Sustained Release Drug Delivery Therapy. These systems leverage the properties of various polymers to control the release rate of the active pharmaceutical ingredient (API). Examples include biodegradable polymers like poly(lactic-co-glycolic acid) (PLGA) and non-biodegradable polymers such as poly(ethylene glycol) (PEG). The choice of polymer depends on factors such as the desired release profile, biocompatibility, and degradation rate. Research in China continues to explore novel biocompatible and biodegradable polymers to improve the efficacy and safety of these systems.
Liposomal Drug Delivery
Liposomal drug delivery systems are another important area of development in China Sustained Release Drug Delivery Therapy. Liposomes are microscopic vesicles composed of lipid bilayers that encapsulate the API, providing sustained release and improved drug targeting. This technology is particularly beneficial in delivering hydrophobic drugs and reducing their systemic toxicity. Many Chinese pharmaceutical companies are actively involved in the research and development of liposomal formulations for various therapeutic applications.
Nanoparticle-Based Systems
Nanoparticle-based drug delivery systems offer significant advantages in China Sustained Release Drug Delivery Therapy, including enhanced drug solubility, targeted drug delivery, and improved bioavailability. These systems utilize nanoparticles made of various materials, such as polymers, lipids, or inorganic materials, to encapsulate and deliver the API. Research focusing on the development of biodegradable and biocompatible nanoparticles for targeted drug delivery is a growing field in China.
Regulatory Landscape and Challenges
The regulatory landscape for China Sustained Release Drug Delivery Therapy is evolving rapidly. The China Food and Drug Administration (CFDA), now the National Medical Products Administration (NMPA), plays a key role in regulating the development and approval of sustained-release drug products. Navigating the regulatory pathways and ensuring compliance with Good Manufacturing Practices (GMP) are crucial aspects for companies involved in this field. Challenges include stringent testing requirements and the need for robust clinical data to demonstrate efficacy and safety. The increasing complexity of these delivery systems also presents significant analytical challenges.
Future Directions and Innovations
Future research in China Sustained Release Drug Delivery Therapy will focus on developing more sophisticated and personalized drug delivery systems. This includes the development of intelligent drug delivery systems responsive to specific stimuli, such as pH or temperature changes, and the use of advanced imaging techniques to monitor drug release in vivo. The integration of nanotechnology, biotechnology, and artificial intelligence is expected to further revolutionize this field, leading to improved therapeutic outcomes and enhanced patient care. Collaboration between academic institutions, pharmaceutical companies, and regulatory bodies will be crucial in driving innovation and ensuring the successful translation of research into clinical practice.
Clinical Applications and Case Studies
China Sustained Release Drug Delivery Therapy is being applied across a wide range of therapeutic areas, including oncology, cardiovascular disease, and diabetes. Successful examples include sustained-release formulations for anti-cancer drugs, which help to improve patient compliance and reduce adverse effects. Further detailed case studies can be found through academic publications and the NMPA databases. Research into the efficacy and safety of these delivery systems across diverse populations remains an active area of investigation.
Table: Comparison of Different Sustained Release Technologies
| Technology | Advantages | Disadvantages |
|---|---|---|
| Polymer-based | Versatile, biocompatible options available | Potential for burst release, complex manufacturing |
| Liposomal | Improved drug solubility, targeted delivery | Stability concerns, potential for aggregation |
| Nanoparticle-based | Enhanced bioavailability, targeted delivery | Toxicity concerns, potential for aggregation |
For further information on cancer treatment and research, consider exploring the resources available at Shandong Baofa Cancer Research Institute. Their commitment to advancing cancer care aligns with the ongoing advancements in China Sustained Release Drug Delivery Therapy.
Disclaimer: This information is for educational purposes only and should not be considered medical advice. Consult with a healthcare professional for any health concerns.
Related products
Related products
Best selling products
Best selling products-
 Mark, a prostate cancer bone metastasis patient from the United States
Mark, a prostate cancer bone metastasis patient from the United States -
 Anthony, lymphocytic cancer patient from the United States 24
Anthony, lymphocytic cancer patient from the United States 24 -
 Andress, a 9-year-old boy from the United States
Andress, a 9-year-old boy from the United States -
 Nell Smith, a throat cancer patient from Switzerland
Nell Smith, a throat cancer patient from Switzerland -
 PAT, rectal cancer patient from the United States
PAT, rectal cancer patient from the United States -
 Famous American female painter Muriel
Famous American female painter Muriel
Related search
Related search- treatment top lung cancer treatment centers cost
- treatment renal cell carcinoma symptoms cost
- hospital do cancer Hospitals
- China stage 1 prostate cancer treatment cost
- China stage one lung cancer treatment Hospitals
- Cheap liver cancer survival near me
- Cheap Baofa Cancer Hospital Hospitals
- China non small lung cancer treatment Hospitals
- China best supplements for prostate cancer treatment
- treatment pi rads 5 prostate cancer treatment Hospitals